With JUVÉDERM® VOLUX™ XC, You Can Receive Non-Surgical Jawline Augmentation in Your Aesthetic Provider's Office
IRVINE, Calif., Jan. 18, 2023 /PRNewswire/ -- Today, Allergan Aesthetics, an AbbVie company (NYSE: ABBV), announced the highly anticipated national launch of JUVÉDERM® VOLUX™ XC. The long-lasting hyaluronic acid (HA) filler is now available at aesthetic practices for consumers over the age of 21 with moderate to severe loss of jawline definition.1
"With 40% of aesthetically-aware consumers considering treatment in the next year to their jawline or jowls with dermal filler,2* Allergan Aesthetics is excited to address this need by offering JUVÉDERM® VOLUX™ XC, a specifically designed and well-researched solution," said Carrie Strom, President, Global Allergan Aesthetics and Senior Vice President, AbbVie. "As the category leader, we continue to innovate by providing aesthetic specialists and patients the broadest portfolio of differentiated fillers."1,3-7
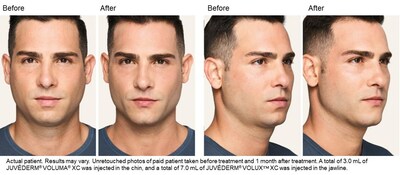
"Many different types of patients can benefit from JUVÉDERM® VOLUX™ XC whether you want to address the appearance of jowls or the contour of the jawline," said Dr. Sachin Shridharani, board-certified plastic surgeon.1 "With the addition of this new dermal filler to the JUVÉDERM® Collection, I can create a smooth, natural-looking, and long-lasting contour that lasts up to twelve months with optimal treatment."1
JUVÉDERM® VOLUX™ XC is the newest member of the JUVÉDERM® Collection, and offers a minimally invasive, non-surgical treatment option with minimal downtime.1 In the JUVÉDERM® VOLUX™ XC clinical trial, patients reported high satisfaction with the results of their treatment.1 The JUVÉDERM® Collection is the number one chosen dermal filler collection in the U.S.8† Additionally, in a survey of aesthetic providers with experience using three or more dermal filler brands, the JUVÉDERM® brand was preferred over two times more than other dermal filler brands.9‡
"The shape of the jawline has a profound effect on an individual's appearance,10 which makes treating this area challenging and requires the practitioner to have advanced skills and precision," said Dr. David Shafer, double board-certified plastic surgeon and Allergan Medical Institute (AMI) trainer. "With the approval of JUVÉDERM® VOLUX™ XC, the AMI curriculum has expanded to include in-depth training, robust clinical trial data, and resources backed by science to ensure that providers have the proper knowledge and technique required to safely treat appropriate patients to meet their aesthetic goals."
Commonly reported side effects in the clinical study included tenderness, lumps/bumps, pain, swelling, firmness, bruising, redness, itching, and discoloration at the injection sites, as reported in their 30-day daily diaries.1 These side effects are consistent with HA filler injections and were usually mild (causing little discomfort and no effect on daily activities) or moderate (causing some discomfort and effect on daily activities) in severity.1 Most of these side effects went away on their own within two weeks.1
Consumers and new patients who receive aesthetic treatment from the JUVÉDERM® Collection of Fillers, can also enroll in Allē, the Allergan Aesthetics loyalty rewards program to unlock access to curated content, exclusive offers, and personalized rewards that can be used for savings on the Allergan Aesthetics portfolio of products and redeemed at a participating provider's office, subject to applicable program terms and conditions. Allē is the first and only loyalty program in the aesthetics market to also offer consumers the ability to earn points on over 40 non-Allergan Aesthetics treatments and brands.
For more information on the JUVÉDERM® Collection of Fillers, visit Juvederm.com and follow @JUVEDERM on Instagram.
About Allergan Aesthetics
At Allergan Aesthetics, an AbbVie company, we develop, manufacture, and market a portfolio of leading aesthetics brands and products. Our aesthetics portfolio includes facial injectables, body contouring, plastics, skin care, and more. Our goal is to consistently provide our customers with innovation, education, exceptional service, and a commitment to excellence, all with a personal touch. For more information, visit www.AllerganAesthetics.com.
About AbbVie
AbbVie's mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people's lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women's health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook, Instagram, YouTube and LinkedIn.
JUVÉDERM® Injectable Gel Fillers Important Information
APPROVED USES
JUVÉDERM® VOLUX™ XC injectable gel is for deep injection to improve moderate to severe loss of jawline definition in adults over the age of 21.
JUVÉDERM® VOLUMA® XC injectable gel is for deep injection in the cheek area to correct age-related volume loss and for augmentation of the chin region to improve the chin profile in adults over 21.
JUVÉDERM® VOLLURE® XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC injectable gels are for injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. JUVÉDERM® VOLLURE® XC injectable gel is for adults over 21.
JUVÉDERM® Ultra XC injectable gel is also for injection into the lips and perioral area for lip augmentation in adults over 21.
JUVÉDERM® VOLBELLA® XC injectable gel is for injection into the lips for lip augmentation and correction of perioral lines, and for injection into the undereye hollows to improve the appearance of undereye hollows in adults over the age of 21.
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive any JUVÉDERM® formulation?
Do not use these products if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), if you are allergic to lidocaine or the Gram-positive bacterial proteins used in these products, or if you have had previous allergic reactions to hyaluronic acid fillers.
What warnings should my doctor advise me about?
- One of the risks with using dermal fillers is the unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. Most of these events are irreversible.
- If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin, or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
- The use of dermal fillers where skin sores, pimples, rashes, hives, cysts, or infections are present should be postponed, as this may delay healing or make skin problems worse.
- The effectiveness of removal of any dermal filler has not been studied.
What precautions should my doctor advise me about?
- JUVÉDERM® VOLBELLA® XC should only be injected into undereye hollows by doctors who have completed the necessary training for this treatment area. To find a doctor, visit Juvederm.com/find-a-specialist. Doctors who complete the training will be listed with a symbol.
- The safety of these products for use during pregnancy or while breastfeeding has not been studied.
- The safety of JUVÉDERM® VOLUMA® XC has not been studied in patients under 35 years or over 65 years for cheek augmentation, or under 22 years and over 80 years for chin augmentation. The safety of JUVÉDERM® VOLUX™ XC, JUVÉDERM® VOLLURE® XC and JUVÉDERM® VOLBELLA® XC has not been studied in patients under 22 years, and the safety of JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC has not been studied in patients under 18 years.
- The safety and effectiveness of treatment with JUVÉDERM® products in anatomical regions outside of their approved uses have not been established in clinical studies.
- If you have a history of excessive scarring (thick, hard scars) or pigmentation disorders, treatment in these patients has not been studied and may result in additional scars or changes in pigmentation.
- If you are planning other procedures including laser treatments or a chemical peel, there is a possible risk of inflammation at the treatment site if these procedures are performed closely before or after JUVÉDERM® injectable gel treatment.
- Tell your doctor if you are on therapy used to reduce your body's natural defense system (such as steroids, chemotherapy, and medicines to treat autoimmune diseases, HIV, and AIDs), as these may increase your risk of infection; and medications that can prolong bleeding (such as aspirin, ibuprofen, or other blood thinners), as these may result in increased bruising or bleeding at the injection site.
- Avoid applying makeup for 12 hours after treatment and minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment, as these may cause temporary redness, swelling, and/or itching at the injection site.
- JUVÉDERM® VOLUMA® XC was not studied in patients with significant loose skin of the chin, neck, or jaw.
- The effect of JUVÉDERM® VOLUMA® XC injection into the chin on facial hair growth has not been studied.
- Patients who experience skin injury near the site of JUVÉDERM® VOLUMA® XC injection may be at a higher risk for adverse events.
- Tell your doctor if you have already been injected with dermal fillers in the same area as the one(s) you are about to be treated for. This information helps your doctor decide when and whether you should get treatment.
What are possible side effects of treatment?
The most commonly reported side effects with JUVÉDERM® injectable gels were redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA® XC, dryness was also reported.
These side effects are consistent with other facial injection procedures and most will resolve within 30 days. Your doctor may choose to treat side effects persisting longer with antibiotics, steroids, or hyaluronidase (an enzyme that breaks down hyaluronic acid).
As with all skin injection procedures, there is a risk of infection.
To report a side effect with any product in the JUVÉDERM® Collection, please call the Allergan® Product Support Department at 1-877-345-5372. Please also visit Juvederm.com or talk to your doctor for more information.
Products in the JUVÉDERM® Collection are available only by a licensed physician or properly licensed practitioner.
* ASAPS Practice Survey Data, 2019 (N = 331)2
† Based on 2022 Market Research Data3
‡ Based on a quantitative survey fielded in May-June 2022 (n=354). Participants were current injectors of JUVÉDERM® fillers and must have used at least two other aesthetic dermal fillers from two separate aesthetics companies
References
- JUVÉDERM® VOLUX™ XC Patient Label 2022
- Data on File, Allergan, VOLUX Consumer Topline Results, February 4, 2022
- JUVÉDERM® VOLUMA® XC Patient Label 2020
- JUVÉDERM® VOLBELLA® XC Patient Label 2020
- JUVÉDERM® VOLLURE® XC Patient Label 2020
- JUVÉDERM® Ultra Plus XC Patient Label 2020
- JUVÉDERM® Ultra XC Patient Label 2020
- Data on File, Allergan, Dermal Filler AMT, August 2022
- Data on File, Allergan, AGN Corporate Image Report, July 2022
- Braz A, Cazerta de Paula Eduardo C. Reshaping the lower face using injectable fillers. Indian J Plast Surg. 2020;53:207-218
SOURCE AbbVie

Contacts
Investors: Liz Shea, Liz.Shea@AbbVie.com, (847) 935-2211, or Media: Ember Garrett, Ember.Garrett@allergan.com, (714) 246-3525